JIANGSU TIANCHENG GROUP LIMITED
Yaoguan Town, Changzhou City, Jiangsu Province,China
+86-519-88387662
+86-519-85121683
steel_tube
steeltube.china
Tiancheng_Group
josen_dong
It all started when people discovered that Iron can react with Carbon and by controling the process something amazing is happening. This is how we start getting steel from iron.
IRON with around 5% of the Earth’s crust and as the 6th most common element in the universe, iron is a highly abundant and immensely popular metal. Unalloyed iron is an unstable element that easily reacts with the oxygen from the air and forms iron oxide. In order to make it more stable, it is commonly alloyed with other elements to create steel.
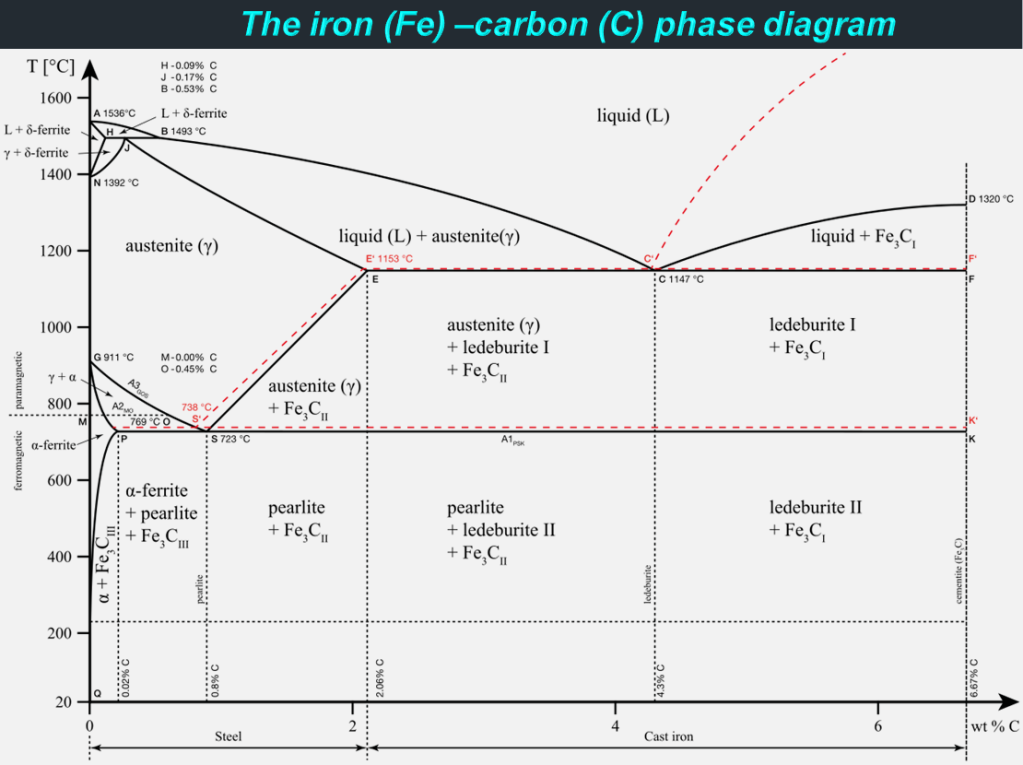
Steel is an iron (Fe) alloy enriched with around 1% carbon (C) and it is generally free of the impurities and residues that can otherwise be found in iron. While iron itself is stronger than other metals, it is remarkably heavy, dense, and prone to corrosion. For these reasons, purely iron structures can be difficult to build and maintain. Not only does adding carbon to iron mitigate these vulnerabilities, but it also makes the material stronger. Additionally, steel has a rather high strength to weight ratio compared to other types of metals, which enables the manufacturing of small yet strong steel parts. With over 3500 different grades and almost 2 billion tons of steel manufactured globally each year, steel is the most commonly used metal in the world. With the number of different elements and varying qualities of those elements being added to create steel alloys there are a multitude of different types of steel. Without further ado, let’s dig deeper into the world of steel!
No one could create stronger and harder steel than the Samurai until the Industrial Revolution. When at this time European countries first started to build structures on a larger, more ambitious scale – such as railways, bridges and ships – they used cast iron, because it could be made in large quantities and poured into moulds. Unfortunately it was extremely prone to fracture under certain conditions. As engineering became more ambitious, so those conditions came about more often.
One of the worst accidents occurred in Scotland. On the night of 28 December 1879, the world’s longest bridge, the cast-iron Tay Rail Bridge, collapsed during extreme winter gales. A train carrying 75 passengers plunged into the River Tay, killing all the passengers. The disaster confirmed what many suspected, that iron just wasn’t up to the job. What was needed was the ability not just to make steel as strong as Samurai swords but to mass-produce it.
One day a Sheffield-based engineer called Henry Bessemer stood up at a meeting for the British Association for the Advancement of Science and announced he had done it. His process didn’t require the elaborate processes of the Samurai. He could create tonnes of liquid steel. It was a revolution in the making.
Henry Bessemer conducted a series of classic experiments for burning off the carbon in the iron by trying various designs of furnace.
The Bessemer Process was ingeniously simple. It involved blowing air through the molten iron, so that the oxygen in the air would react with the carbon in the iron and like that the 4% Carbon already present in the iron would burn and is removed as carbon dioxide gas . It required a knowledge of chemistry that for the first time put steel making on a scientific footing. Moreover, the reaction between the oxygen and the carbon was extremely violent and gave off a lot of heat. This heat raised the temperature of the steel, keeping it hot and liquid. The process was straightforward and could be used on an industrial scale; it was the answer.

The only problem with the Bessemer Process was that it didn’t work. Or at least that was what everyone who tried it said. Soon, angry steelmakers, who had bought the licence from Bessemer and invested large sums of money in equipment only to produce brittle iron, started asking for their money back. He had no answers for them. He didn’t really understand why the process was successful sometimes and unsuccessful at others, but he continued to work on his technology, and with the help of the British metallurgist Robert Forester Mushet he adapted his technique. Rather than trying to remove the carbon until just the right amount was left, about 1%, which was tricky because each steelmaker had a different source of iron, Mushet suggested removing all the carbon and then adding 1% carbon back in. This worked and was repeatable.
These critical advancements made the iron and steel industry flourish. This was especially evident in the railways where the speed, weight, and quantity of railway traffic was limited by the strength of wrought iron rails. Switching to steel thus positively transformed the transport sector, due to their greater strength and durability and ability to handle the increasingly heavy and faster cars and engines. This led to the mushrooming of many other manufacturing activities dependent on steel and/or transportation. It would then be safe to reason that iron and steel effectively fuelled the industrial revolution. Europe, and Britain in particular, was the pivot of this revolution largely because of their large supply of coal and iron ore. After 1890, the Bessemer process was gradually supplanted by open-hearth steel-making. The crucible process further enhanced the production of high-quality alloy steel in the 20th century.
Of course, when Bessemer tried to interest the world in this new process, the other steelmakers ignored him, assuming that it was yet another swindle. They insisted that it was impossible to create steel from liquid iron, and Bessemer was a con artist. There are many stories of those who have laughed at visionaries only to be embarrassed by their subsequent success. Many laughed at the idea that heavier-than-air flying machines were possible, and yet we all fly around in them, Likewise, television, mobile phones, computers- all have emerged from a cloud of derision.
In the end he saw no option but to set up his own steel works, and just start making the stuff himself. After a few years the firm of Henry Bessemer & Co. was manufacturing steel so much more cheaply and in such larger quantities than his rival firms that they were eventually forced to license his process, in the end making him extremely rich and ushering in the machine age.
Another great invention related to steel at the beginning of 20th century was the razor blades and surgical instruments. Until the 20th century, steel razors and surgical knives were extremely expensive. They had to be hand-made from the highest-grade steel since only this type of steel could be sharpened sufficiently to cut facial hair cleanly and effortlessly, without snagging. (Anyone who has used a blunt razor will know all too well how acutely painful even the slightest snag can be.) And because steel corrodes in the presence of air and water, cleaning the blades blunts them too, as the fine cutting edge literally rusts away. Thus for thousands of years the ritual of shaving began with the process of ‘stropping’: the act of sharpening the blade by playing it back and forth along a length of leather. You might think it not credible that a material as soft as leather can sharpen steel, and you would be right. It is the fine ceramic powder that is impregnated in the leather strop that does the sharpening. Traditionally a mineral called ‘jewellers’ rouge’ was used, but these days diamond powder is more common. The act of running the steel along the strop, in a flip-flop manner, causes the blade to meet the extremely hard particles of diamond that are in the powder, which remove very small amounts of metal in the collision, causing the delicately fine cutting edge to be restored.
But this changed when, in 1903, an American businessman called King Camp Gillette decided to use the new cheap industrial steel produced by the Bessemer Process to create a disposable razor. This was to be the democratization of shaving. His vision was to remove the need to sharpen the blade by making it so cheap that when it became blunt you could simply throw it away.
In 1903 Gillette sold 51 razors and 168 blades. The folIowing year, he sold 90,884 razors and 123,648 blades. By 1915, the corporation had established manufacturing facilities in the United States, Canada, England, France and Germany, and razor blade sales exceeded 70 million. Once people stopped needing to go to the barber’s for a shave, the disposable steel razor became a permanent fixture of every bathroom. And it has remained so: while there are any number of back-to-basics movements in food production, no one wants to have their hair cut with a copper knife or a shave with a blunt razor.

Gillette’s business model was clever for many reasons, one of which was undoubtedly that even if the razors were not blunted through the act of shaving they would lose their edge quickly through rusting, assuring repeat business. But there was one further twist to the tale, an innovation so outrageously simple that it had to be discovered by accident.
In 1913, as the European powers were busily arming themselves for the 1st World War, Harry Brearley (an English metallurgist credited with the invention of “stainless steel”)had the job of investigating metal alloys in order to create improved gun barrels. He was working in one of Sheffield’s metallurgy labs, adding different alloying elements to steel, casting specimens and then mechanically testing them for hardness. Brearley knew that steel was an alloy of iron (Fe) and carbon (C), and he also knew that lots of other elements could be added to steel to improve or destroy its properties. No one at the time knew why, so he proceeded by trial and error, melting steels and adding different ingredients in order to discover their effects. One day it was aluminium, the next it was nickel.
Brearley made no progress. If a new specimen turned out not to be hard, he chucked it in the corner. His moment of genius came when a month later he walked through the lab and saw a bright glimmer in the pile of rusting specimens. Rather than ignoring it and going to the pub, he fished out this one specimen that had not rusted and realized its significance: he was holding the first piece of stainless steel the world had ever known. Accidentally, by getting the ratios of two alloy ingredients right, carbon and chromium, he had managed to create a very special crystal structure in which the chromium and carbon atoms were both inserted inside the iron crystals. The addition of chromium had not made the steel harder, hence he had rejected the sample, but it had done something much more interesting.
Normally when steel is exposed to air and water, the iron on the surface reacts to form iron(III) oxide Fe2O3, a red mineral commonly known as rust. When this rust flakes off, it exposes another layer of the steel to further corrosion, which is what makes rusting such a chronic problem for steel structures, hence the need to paint steel bridges and cars. But with chromium present something different happens. Like some hugely polite guest, it reacts with the oxygen before the host iron atoms can do so, creating chromium oxide Cr2O3. Chromium oxide is mineral, it is transparent and hard and sticks extremely well to the steel. In other words it doesn’t flake off and you don’t know it is there. Instead it creates an invisible chemically protective layer over the whole surface of the steel. What’s more, we now know that the protective layer is self-healing, which is to say that when you scratch stainless steel, even though you break the protective barrier, it re-forms.

Brearley went on to try to make the world’s first stainless steel knives, but immediately ran into problems. The resulting metal was not hard enough to make a sharp edge, and they were, soon dubbed ‘knives that would not cut’. This lack of hardness was, after all, the very reason that Brearley had rejected the alloy for use in gun barrels. Its lack of hardness allowed the alloy to do other things, though, which only became apparent later in the century – namely, it could be formed into complex shapes, leading eventually to one of the most influential piece of British sculpture, present in almost every house: the kitchen sink.
Stainless steel sinks are indomitable and gleaming and seem able to take anything that is thrown at them, In a world where we are keen to dispose of waste instantly and conveniently-from fat, to bleach, to acid – this material has really come-through for us. It has ousted ceramic sinks from the kitchen, and would oust the ceramic bowl from the toilet if we would let it, but we do not yet trust this new metal quite enough for that most intimate of waste disposal jobs. Stainless steel is the very epitome of our modern age. It is clean-looking and shiny, appears almost indestructible but is ultimately very democratic: in less than a hundred years it has become the metal with which we are the most closely acquainted; after all, we put it in our mouths almost every day.
For, in the end, Brearley did manage to create cutlery from stainless steel, and it’s the transparent protective layer of chromium oxide that makes the spoon tasteless, since your tongue never actually touches the metal and your saliva cannot react with it; it has meant that we are one of the first generations who have not had to taste our cutlery. It is often used in architecture and art precisely because its bright surface appears uncorrodable.
By solving the problem of making stainless steels hard enough for cutlery, metallurgists also unwittingly solved the problem of razors rusting, thus creating the finest cutting blade the world has ever known, and in the process altering the appearance of so many faces and bodies. Inadvertently, this domestication of shaving has also created a weapon of choice for street crime: razors that are both durable and cheap, but more than that, ultra-sharp – able to slice through several layers of leather, wool, cotton and skin.
As stainless steel, a hard, tough, sharp steel impervious to water and air, has been created mostly through trial and error over the last few thousand years, it didn’t seem utterly impossible that someone, even without a scientific training, might yet stumble across a process for resharpening a razor blade. The microscopic world of materials is so complex and huge that only a fraction of it has been explored.
Stainless steel is therefore a highly corrosion-resistant alloy steel that consists of iron, carbon, significant amounts of chromium and residues of other metals. It is a versatile material widely applied in many households. From kitchen utensils to tables, sinks, and other furniture, stainless steel is perfect for the manufacturing of anything that comes into contact with food because it doesn’t rust easily. Iron finds its application in cookware because its porous surface combined with hot oil prevents sticking. Due to its remarkably high melting point, cast iron is used for the production of wood stoves. Being a heavy metal, iron provides rigidity and reduces vibrations, which is why it is often used for the manufacturing of heavy machinery frames and bases.
Besides Stainless Steel some other very important and useful version of steel as possible as well.We ca also get the following 3 main categories of steel:
I. Alloy Steel= made by combining elements such as chromium (Cr), manganese (Mn), nickel (Ni), tungsten (W), or vanadium (V) with iron (Fe). Each of the alloying elements brings different properties to the mix, thus making the alloy steel highly customizable. Depending on the needs of the project, the specific alloy can be modified to produce many desired qualities, a couple of which might be a higher material strength or a product that is more resistant to wear and corrosion. Alloy steel can be relatively inexpensive to produce, making it very widely used.
II. Carbon steel = made of iron and carbon, sometimes with residues of other elements. It is commonly categorized into three groups (low, medium, and high carbon steel) based on the amount of carbon the alloy contains. The more carbon is used to manufacture steel, the harder the alloy will be. On the other hand, small amounts of carbon make for an alloy that is easier and cheaper to manufacture. Carbon steel is often used to produce tools and mechanical elements but is best known as a structural building material.
III. Tool Steel = Due to its hardness, tool steel is used for the manufacturing of cutting, drilling, and other shock-resistant tools. The hardness comes from alloying iron with elements such as cobalt (Co), molybdenum (Mo), tungsten (W), or vanadium (V). Tool steel has a wide variety of applications, including construction, shipbuilding and automotive industries. It is primarily used to machine and make changes to different kinds of steel.
Today, China is among the largest producers and consumers of steel worldwide. As much as it is strong, steel is also fully recyclable, which makes it a potent candidate to propel a sustainable present and future.